
About Us
About NBM
NatureWise Biotech & Medicals Corporation (NBM), a new drug development company founded in the year of 2000, focuses on the botanical drug and small molecule therapies. In order to strengthen research capabilities, new drug R&D center was established in 2004 located in the NanKang Biotech Incubation Center (NBIC) of the Ministry of Economic Affairs. Our first product, LipoCol Forte®, is the first traditional Chinese Medicine underwent clinical trial and approved by the Department of Health. Via collaborated with academic institutions, we invest in the development of therapeutic drugs for unmet medical needs. Several projects are in progress and will start the clinical trial.
Our Strategy
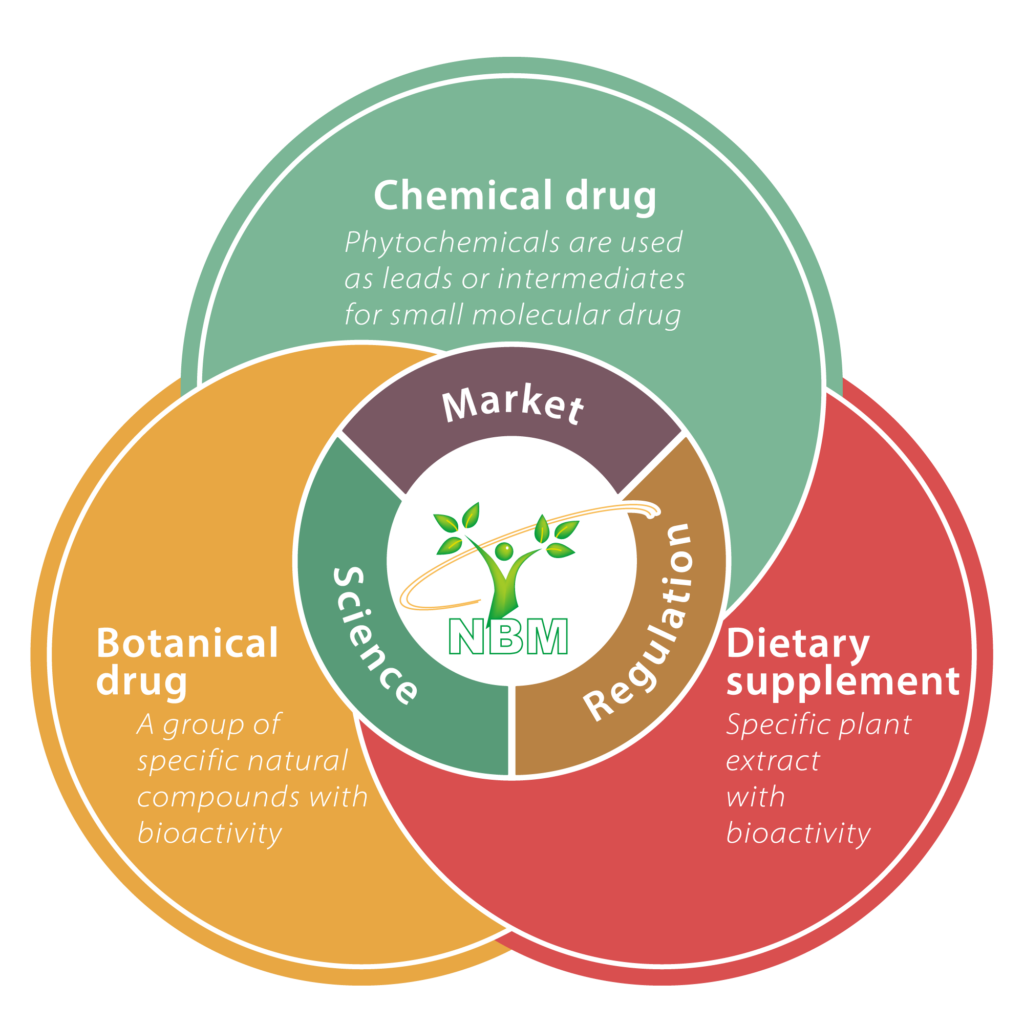
By taking advantage of our profound knowledge in herbal medicines, we taken natural plants as the development target and started from the niche market to systematically develop the new value of natural plants. Based on the comprehensive evaluation on market, regulatory, and science, we draw up the best development strategies of drug candidates to be used in diseases in need of better treatment modalities.
R & D
Products
NBM-BMX
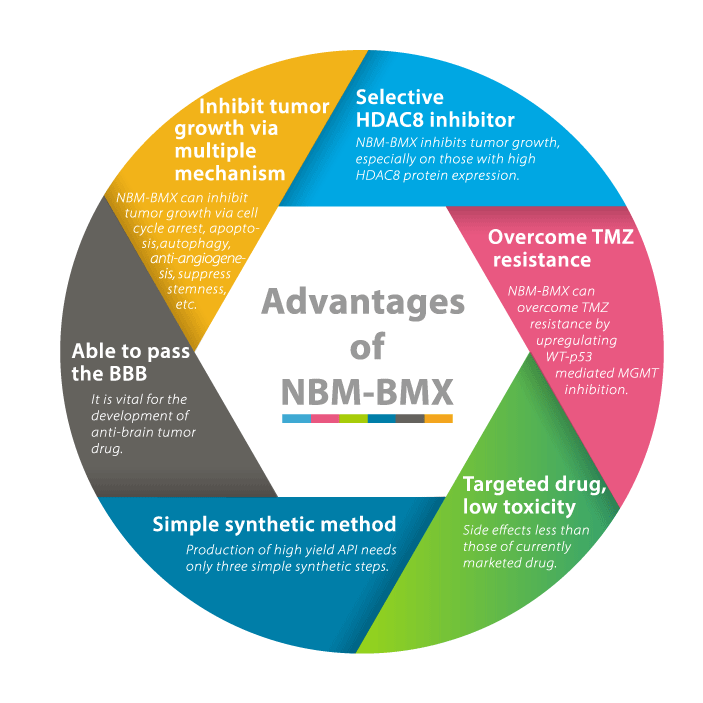
NBM-BMX is a new chemical entity developed by NatureWise Biotech & Medicals Corporation. It is the first specific histone deacetylase 8 (HDAC8) inhibitor in the world that has undergone clinical trials in humans. The development of isoform-selective HDAC inhibitors is becoming important in recent years due to the side effects observed in non-selective HDAC inhibitors.
NBM-BMX exhibited tumor inhibitory activity against many cancers, especially those with high HDAC8 protein expression. NatureWise owns the global rights and patents to NBM-BMX. Two Phase 1 clinical trials have been completed in both the USA and Taiwan with good safety and significant efficacy results. The phase Ib/II clinical trials for newly diagnosed glioblastoma multiforme begins in 2023.
LipoCol Forte® Capsule
LipoCol Forte® is the first traditional Chinese medicine underwent clinical trial in accordance with “Good Clinical Practice” and approved in Taiwan to lower cholesterol and triglyceride. LipoCol Forte® is derived from Monascus purpureus Went fermented on premium rice. The active constituent of LipoCol Forte® can inhibit HMG-CoA reductase, the rate-controlling enzyme in the cholesterol synthesis pathway. It is indicated for hypercholesterolemia and hypertriglyceridemia.
PPLs® Taiwan Green Propolis
PPLs® are a group of prenylflavanones with HDAC inhibitor activity isolated from Taiwan green propolis by a unique separation technology. NatureWise has undertaken studies of Taiwan green propolis for many years, and the study results received many patents and awards. PPLs® can increase the survival of neural stem cells, enhance the outgrowth of neurites, and modulate blood sugar.

NEWS
NBM-BMX received USFDA approval to proceed Phase Ib/II clinical trial in newly diagnosed glioblastoma multiforme (GBM).
NatureWise’s new drug, NBM-BMX, received USFDA approval on January 4th, 2023 to proceed in Phase Ib/II clinical trials in newly diagnosed GBM. Glioblastoma multiforme is one of the most aggressive, invasive, and deadly malignancies. More than 60% of newly diagnosed GBM patients do not respond to standard treatment. Our studies demonstrated that NBM-BMX is a new therapeutic approach with the potential for addressing this need for safer, more effective treatment options for GBM.
The USFDA has reviewed and commented on the results of the phase 1 clinical trial of NBM-BMX and suggested changes on the draft clinical protocol of the phase 2 program
In 2018, in a letter approving the initiation of phase 1 clinical trials, the USFDA requested NatureWise schedule an end of phase 1 (EOP1) meeting with the FDA when NatureWise completed the phase 1 clinical program. In September 2021, NatureWise completed a phase 1 clinical trial in the USA, submitted the trial results to the USFDA along with a draft of the phase 2 [...]



